*** Advanced Project Notice ***
The following project is an ADVANCED project and may require handling of dangerous materials and/or equipment and is intended to be conducted by adults only!
Purpose
To demonstrate the ability of oxygen and carbon monoxide to bind with haemoglobin.
Additional information
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of vertebrates, and the tissues of some invertebrates. Oxygen binding to haemoglobin is a reversible reaction. At lungs oxygen binds to haemoglobin to form the oxygen-haemoglobin complex called oxyhaemoglobin. Once these oxyhaemoglobins are transported into tissues they release oxygen for cellular respiration. After this, the haemoglobins are available to bind with oxygen again at lungs. But carbon monoxide binds to haemoglobin irreversibly and 200 times faster than oxygen to form a very bright red compound called carboxyhaemoglobin, meaning that small amounts of CO dramatically reduce hemoglobin's ability to transport oxygen. This incident can even bring death to individuals. When inspired air contains CO levels as low as 0.02%, headache and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen-active sites can be blocked by CO.
In similar fashion, hemoglobin also has competitive binding affinity for cyanide (CN-), sulfur monoxide(SO), nitrogen dioxide (NO2), and sulfide(S2-), including hydrogen sulfide (H2S). All of these bind to iron in heme without changing its oxidation state, but they nevertheless inhibit oxygen-binding, causing grave toxicity.
Haemoglobins absorb electromagnetic radiation in visible range. The wave lengths at which a maximum absorbance is showed depends on the bound ligand. By plotting a graph, absorbance vs. wave length we can identify haemoglobin (no bound ligand), oxyhaemoglobin and carboxyhaemoglobin.
Sponsored Links
Required materials
- Centrifuger
- Colorimeter (Spectrophotometer-visible range)
- 15ml Test tubes
- Glass/rubber tubes
- 1 ml of human/animal blood sample
- Sodium citrate buffer (pH 7.0)
- Deionized water
- 0.9% NaCl solution
- Stokes’ reagent –Dissolve 3g of ferrous sulphate in a small quantity of water and add to it in watery solution 2g of tartaric acid. Make up the mixture to 100ml and just before using add NH4OH until the precipitate which at first forms is dissolved.
Estimated Experiment Time
1-2 hours
Step-By-Step Procedure
- A. Haemoglobin Isolation
- 1. Collect a blood sample from a human/animal.
- 2. Transfer 1ml of blood into 5ml of citrate buffer (to prevent clotting).
- 3. Centrifuge the mixture at 3000g*1 for 5 min.
- 4. Remove the supernatant plasma.
- 5. Add 10ml of 0.9% NaCl solution to the bottom cellular sediment and mix gently.
- 6. Centrifuge the sample again at 3000g for 5min.
- 7. Remove the supernatant again.
- 8. Repeat steps 5-7, to wash the red cells until the supernatant is colorless.
- 9. Resuspend the washed red blood cells in 2 volumes of deionized water and centrifuge at 10000g for 10 min.
- 10. Decant the red supernatant and use it for the following tests.
- B. Oxyhaemoglobin spectrum
- 1. Dilute a sample of haemoglobin using the buffer solution. (The dilution depends on the absorbance values. Dilute the sample until the absorbance values are less than 1.0. You have to figure out the dilution by doing trials).
- 2. Shake the sample in air for a while (to form oxyhaemoglobin complexes)
- 3. Measure the absorption spectrum between 400-700nm, using the colorimeter*2. (If necessary dilute the sample again).
- 4. Plot a graph absorbance vs. wave length.
- C. Deoxyhaemoglobin (haemoglobin with no bound oxygen)
- 1. Prepare a diluted sample of haemoglobin.
- 2. To remove bound oxygen if there any, add 2 drops of freshly prepared Stoke’s reagent to 4ml of haemoglobin solution.
- 3. Obtain the absorbance spectrum as in procedure B.
- D. Carboxyhaemoglobin spectrum
- 1. Get a diluted sample of haemoglobin solution.
- 2. Bubble CO gas through the sample.
- i. CO is found in vehicle emission. Use the FIGURE A set to mix vehicle emission with haemoglobin.
- ii. Attaché the funnel/funnel shaped tube to the exhaustion pipe of a started vehicle.
- iii. Bubble the haemoglobin solution for 1-2 min.
- 3. Obtain the absorbance spectrum as in procedure B.
Note
*1 - Relative centrifugal force (RCF) expressed in units of gravity (times gravity or x?g). Many microcentrifuges only have settings for speed (Revolutions per minute, RPM), not relative centrifugal force. Consequently, a formula for conversion is required to ensure that the appropriate setting is used in an experiment. The relationship between RPM and RCF is as follows:
g = (1.118 ??10-5) R S2
Where g is the relative centrifugal force, R is the radius of the rotor in centimeters, and S is the speed of the centrifuge in revolutions per minute.
*2 – for colorimetric measurements, use the buffer used to dilute the haemoglobin sample as the zero dilution sample. If you are not familiar with colorimeter better use some guidance from a technician. As this is an expensive instrument, be cautious.
Figures & Illustrations
| Figure 1 |
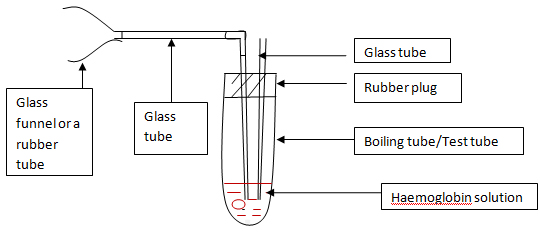 |
Observation
You will get absorbance spectra for haemoglobin(deoxyhaemoglobin), oxyhaemoglobin and carboxyhaemoglobin similar to following figure.
| Figure 2 |
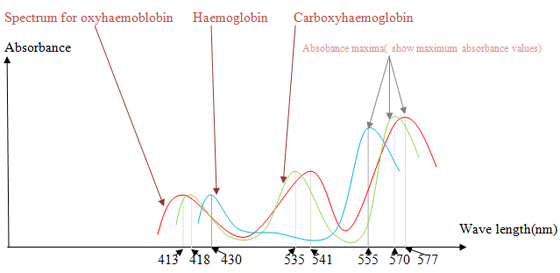 |
| Figure 3 |
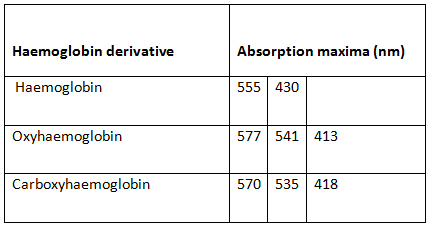 |
Result
See Observation
Sponsored Links
Take a moment to visit our table of Periodic Elements page where you can get an in-depth view of all the elements,
complete with the industry first side-by-side element comparisons!